Atoms are very, very small. With a nucleus of
protons (or protons and neutrons) and orbiting
electrons, atoms make up the fundamental elements of
the Universe. While atoms can be broken down to
their own fundamental parts, elements like Hydrogen
or Sulfur cannot be separated to "lesser" elements -
thus are fundamental.All of the elements make up
what we call the Periodic Table of Elements. This
tool describes in detail information about each
element such as how many components of protons,
neutrons and electrons are.
The best source for an online Periodic Table of
Elements is
Web Elements.
A molecule is a collection of elements. An
example is a water molecule: H2O - two
parts hydrogen, one part oxygen.
More information on the specifics of the elements
is found on the
Web Elements website. The purpose of this
section is to visit a typical atom. We will briefly
visit:
Energy Levels
Ionization
Isotopes
Energy Levels:
| An atom consists of at least one part, a
proton. This positively charged particle
resides at the center of the atom. The
atomic weight of an element is dependant on
how many protons an atom has.
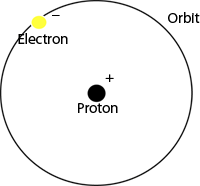
This is a model of the Bohr Atom, named
after Niels Bohr. The Bohr model is designed
to demonstrate how absorption and emission
lines appear in spectra taken from the Sun.
|
 |
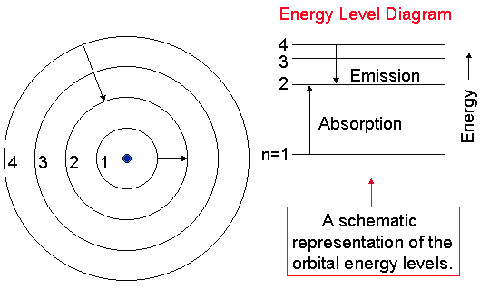
An atom consists of several possible orbits for
the electron. The orbit depends on the energy of the
electron:
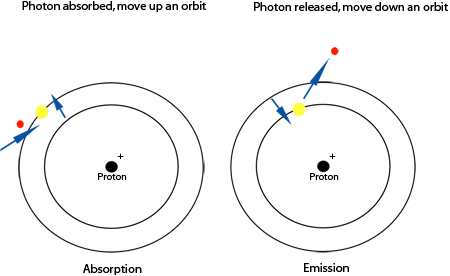
When an electron absorbs a photon (a packet of
light), the electron gains energy and moves up an
orbit. The image above demonstrates a hydrogen atom
with 4 possible orbits (there can be more). Only one
electron can be in one orbit at one time (actually,
two electrons can occupy an orbit as long as they
have different "spin" - see the
Quantum Physics
section). When an electron gains energy and moves
up an orbit, an absorption spectra occurs. When an
electron looses energy and moves back down an orbit,
an emission spectra occurs (see
Spectroscopy).
These energy levels follow a pattern:
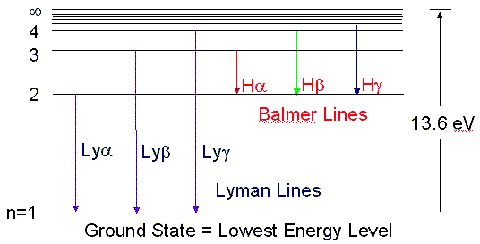
There are actually three specific series of emission
lines:
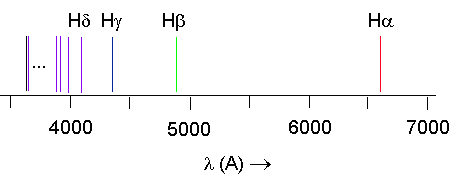
- The Balmer series - emission that occurs
within the visible spectrum
- The Lyman series - emission that occurs
within the ultraviolet spectrum
- The Paschen series - emission that occurs
within the infrared spectrum
Here is a look at the frequencies of the
Balmer lines:
Back to Top
Ionization:
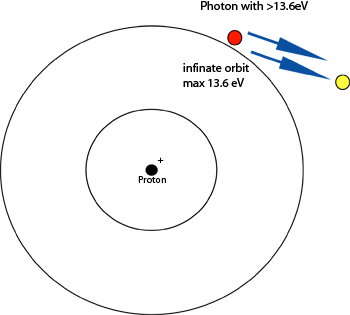
Sometimes if the energy of the photon absorbed
by the electron is greater than the allowed
energy levels of the orbits, the electron will
be striped away by that
photon. This is called
ionization. For the hydrogen atom, the infinite
orbit has an allowed energy of 13.6eV. If a
photons energy is greater than this, ionization
occurs:
With regard to hydrogen, a hydrogen atom in its
normal state is also called H I. A hydrogen atom
that has been stripped of its electron is also
called H II. Astronomers use H I and H II to
help study interstellar debris and dust clouds.
Back to Top
Isotopes: An
isotope is an element (an atom) that has
additional neutrons. The Hydrogen atom, that is
your standard hydrogen complete with electron,
only has a proton. However, hydrogen can have
added to it a neutron. A neutron is a particle
that has no charge, so it does not add to the
atomic weight. A hydrogen atom that has one
neutron is called Deuterium.
Deuterium = 2H
According to
Wikipedia deuterium is a naturally occurring
element - approximately 1 out of every 6500
hydrogen atoms is deuterium. Deuterium is
considered a stable isotope, in that the neutron
will not decay. Any element that looses neutrons
is unstable and is said to be decaying - also
know as
radioactivity.
Back to Top
|

