| We
often associate
temperature with how hot or cold
something is. If we see a piece of metal glowing
red, that would be hot to the touch. A ground
covered with snow would be cold to touch. This is
only part of the story. In
physics,
temperature is
the measure of how internal particles move.
The image above shows an example of this. If this
image represents a gas, the particles that
constitute the gas would move depending on
temperature. In a hot gas, the molecules
(represented by the dots) are free to move - the
faster they move, the hotter the gas. Alternately, a
cold gas would equal slow or very minimal movement
of molecules. Absolute zero is defined as
molecules in a medium that are not moving at all. In
the Celsius
temperature scale, absolute zero is
-273.2 degrees C. In an effort to standardize the
temperature scale, Kelvin's are preferably used as 0
(zero) on the Kelvin scale is absolute zero. As
such:
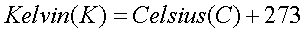 Celsius has by no means been
abandoned. Much of our standards are based on the
Celsius scale:
The Fahrenheit scale is used
prominently in the United States, and mostly for
weather and cooking measurements. This scale is not
at all used in science.
The
temperatures above do not follow
a nice neat pattern like the Celsius scale, but of
special note:
-40 degrees Fahrenheit = -40
degrees Celsius
To convert Fahrenheit to Celsius:
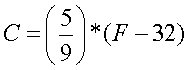
Other important notes:
The symbol for degrees (o) or the
word degree is NOT used on the Kelvin scale:
Absolute zero = 0 K
The symbol for degrees or the word
degree IS to be used for Celsius or Fahrenheit
temperature scales.
In science, DO convert Fahrenheit to
Celsius and avoid using Fahrenheit altogether.
Back to Top
|

