| The electromagnetic spectrum (also called the EM-band) contains
gamma rays,
radio waves and everything in
between. The history of discovering the
electromagnetic spectrum is a fascinating
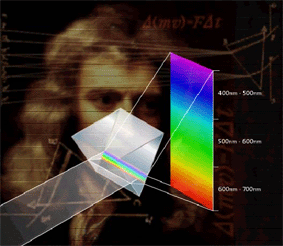
one. Sir Isaac Newton used a prism to split
sunlight into its fundamental colors of the
rainbow. |
 |
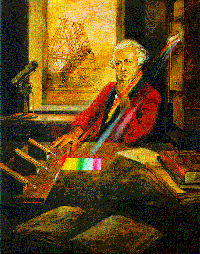 |
Sir William Herschel discovered, by
accident, the infrared portion of the
spectrum when he placed thermometers above
the red portion of a projected spectrum. The
image on the left shows how this was
confirmed, with one of the three
thermometers places above the red portion of
the spectrum |
Johann Ritter suspected "invisible" light on the
opposite (blue) end of a spectrum and used paper
soaked with silver chloride to detect it. Thomas
Young confirmed the wave
nature of light (that light
moves like waves similar to ocean waves) by
examining diffraction patterns through slits.
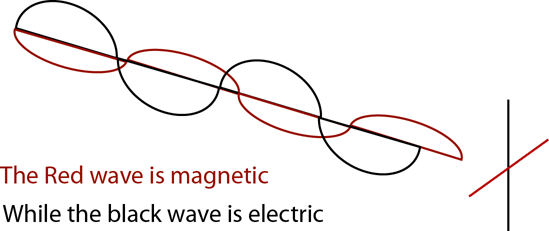
Michael Faraday and James Maxwell collaborated
together to theorize the electromagnetic
nature of light - that
is changing the electric current in the wave alters its
magnetic field. The image below (don't laugh, I'm
still learning Adobe Illustrator!) demonstrates how
the magnetic portion of this wave is 90o
to the electric portion of the wave - hence 'electromagnetic.'
Experimenting with "Maxwellian Waves," Heinrich
Hertz discovered radio waves. Wilhelm Rontgen
discovered X-Rays while seemingly serendipitously
experimenting with electric current through
cardboard tubes with exposed film he had on the other end
of the room - he saw the bones of his hand.
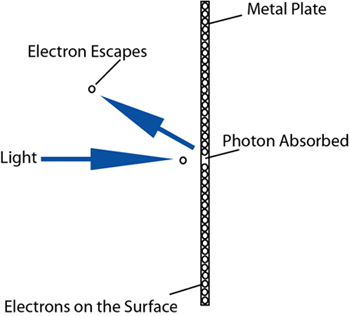
It wasn't until Albert Einstein, winning the Nobel
Prize for discovering the photoelectric effect, that
the wave-particle duality of electromagnetic waves was
understood.
Eventually, a tool was created in the form of the
electromagnetic spectrum.
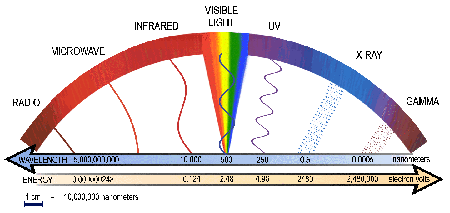
As a starting point for understanding the spectrum,
visible
light covers only a small part - 400
nanometers (blue) to 700 nanometers (red). The image below is a
graph from the
Chandra website demonstrating the entire
electro-magnetic spectrum. Notice that only a very
small portion of this spectrum (in the middle) is
made up of visible
light.
Back to Top
|

