|
The term Quantum Physics strikes fear into
non-scientist major when required to take a
Physics
course - but its really not that bad.
Quantum
Physics is nothing more than the study of
fundamental particles and their interactions with
one another. To start, there are two wonderful
websites that contain information on just about
everything you could want to know about fundamental
particles:
The following images are from the Particle Adventure
website, but also available through the Stanford
site.
Basically, everything in the Universe falls into one
of two fundamental categories:
This theory of fundamental particles is
called The Standard Model.
In addition, every particle has a
corresponding anti-particle. This may sound
totally science-fiction, but the premise is
really quite simple. Particles have a particular
spin, and its corresponding anti-particle will
have the opposite spin - nothing more.

The image above demonstrates the fundamental
particles that fall under the category Fermions. The
name Fermion comes from Enrico Fermi. The Fermions
category obeys the Pauli Exclusion Principle which
states no two fundamental particles can occupy the
same quantum state. This means that two "up quarks"
cannot be in the same space.
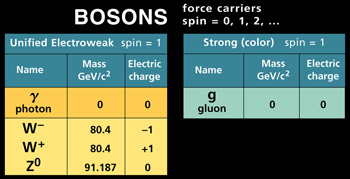
Bosons on the other hand can occupy the same
quantum state with one another. While not
particles per se, Bosons are force carriers -
they carry energy , like the
photon.
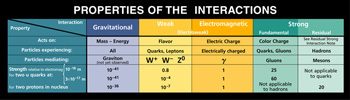
And speaking of force carriers, there are 4
fundamental forces:
-
Gravity
-
Weak
-
Electromagnetic
-
Strong
The Strong force is what keeps
the
atomic nucleus together. The weak and
electromagnetic force together for the
"electroweak" force. As far as Gravity, the
theory is that the particle involved with this
is called the Graviton, but this particle has
yet to be seen.
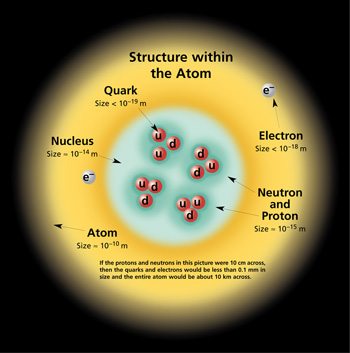
The above image of the
atomic
structure should help put this in perspective.
The nucleus of this particular atom contains two
protons (2 up quarks and 1 down quark) and two
neutrons (2 down quarks and 1 up quark). These
would be held together by the Strong force. The
electron is already a fundamental particle.
There are other fundamental
particles, but I will leave that up to you. This
information here is enough to introduce the
premise of Quantum Physics.

The image above shows the
complete poster of fundamental particles.
Image Credits
Back to Top
|

